By James E. Lovelock, Bowerchalke, Nr. Salisbury, Wilts. England and Lynn Margulis, Department of Biology, Boston University, 2, Cummington Street, Boston, Mass., USA.
Published in Tellus XXVI (1974), 1-2. Manuscript received May 8 1973; revised version August 20, 1973.
Abstract. During the time, 3.2 x 109 years, that life has been present on Earth, the physical and chemical conditions of most of the planetary surface have never varied from those most favourable for life. The geological record reads that liquid water was always present and that the pH was never far from neutral. During this same period, however, the Earth’s radiation environment underwent large changes. As the sun moved along the course set by the main sequence of stars its output will have increased at least 30% and possibly 100%. It may also have fluctuated in brightness over periods of a few million years. At the same time hydrogen was escaping to space from the Earth and so causing progressive changes in the chemical environment. This in turn through atmospheric compositional changes could have affected the Earth’s radiation balance. It may have been that these physical and chemical changes always by blind chance followed the path whose bounds are the conditions favouring the continued existence of life. This paper offers an alternative explanation that, early after life began it acquired control of the planetary environment and that this homeostasis by and for the biosphere has persisted ever since. Historic and contemporary evidence and arguments for this hypothesis will be presented.
It is widely believed that the abundance of the principal gases N2 and O2 is determined by equilibrium chemistry. One of the larger problems in the atmospheric sciences is that of reconciling this belief with the uncomfortable fact that these same gases are cycled by the Biosphere with a geometric mean residence time measured in thousands of years. The more thoroughly the inventory of an individual gas is audited the more certain it seems that inorganic equilibrium or steady state processes determine its atmospheric concentration but the same audit frequently further reveals the extent of its biological involvement. A lucid account of contemporary information on the problem of the cycle of gases is in the paper of Junge (1972).
This paper presents a new view of the atmosphere, one in which it is seen as a component part of the biosphere rather than as a mere environment for life. In this new context the incompatibilities of biological cycles and inorganic equilibria are seen as more apparent than real.
A starting point is a consideration of the profoundly anomalous composition of the Earth’s atmosphere when it is compared with that of the expected atmosphere of a planet interpolated between Mars and Venus. Thus on Earth the simultaneous presence of O2 and CH4 at the present concentrations is a violation of the rules of equilibrium chemistry of no less than 30 orders of magnitude. Indeed so great is the disequilibrium among the gases of the Earth’s atmosphere that it tends towards a combustible mixture, whereas the gases of Mars and Venus are close to chemical equilibrium and are more like combustion products.
The anomalous nature of the atmosphere has been known since Lewis & Randall (1923) first commented that at the pE and pH of the Earth the stable compound of nitrogen is the N03- ion in the oceans; gaseous nitrogen should not be present. In spite of reminders by Hutchinson (1954) and Sillén (1966) this anomaly has remained unnoticed in the debate on atmospheric cycles.
Given the chemical composition of a planetary atmosphere it is possible to infer the presence or absence of life, Hitchcock & Lovelock (1967). To do this the entire ensemble of reactive gases constituting the atmosphere needs be considered and when this is done information is made available which is otherwise inaccessible when each gas is considered separately in isolation. This approach applied to the present problem of the anomaly of the chemical distribution of the gases of the atmosphere, offers a strong suggestion that the Earth’s atmosphere is more than merely anomalous; it appears to be a contrivance specifically constituted for a set of purposes.
This paper examines the hypothesis that the total ensemble of living organisms which constitute the biosphere can act as a single entity to regulate chemical composition, surface pH and possibly also climate. The notion of the biosphere as an active adaptive control system able to maintain the Earth in homeostasis we are calling the ‘Gaia’ hypothesis, Lovelock (1972). Hence forward the word Gaia will be used to describe the biosphere and all of those parts of the Earth with which it actively interacts to form the hypothetical new entity with properties that could not be predicted from the sum of its parts.
Theoretical basis
The fundamental problem underlying the formal recognition of an unfamiliar living association, such as Gaia, is that of recognising life itself. We are so preprogrammed to recognise life instinctively that the logical basis of the recognition is rarely questioned. Thus the discovery of a member of new and bizarre animal species raises the question, what is it, never the question, is it life? This instinctive recognition passes beyond organisms to systems provided that they are not too big to be seen. Thus a bees nest is recognised as a purposeful structure, quite different from a cluster of non-social insects. The intricate organisation of an ecosystem such as a tropical rain forest, however, was not recognised until the evidence of the interdependence of its parts and the economy of the cycling of essential elements was discovered. With the total ecosystem, Gaia, apart from the shadowy evidence of those primitive beliefs in her reality we are dependent upon physical rather than biological evidence. There is little doubt that living things are elaborate contrivances. Life as a phenomenon might therefore be considered in the context of those applied physical sciences which grew up to explain inventions and contrivances, namely thermodynamics, cybernetics and information theory.
The first cautious approach to a classification of life, reached general agreement as follows:
‘Life is one member of the class of phenomena which are open or continuous reaction systems able to decrease their entropy at the expense of free energy taken from the environment and subsequently rejected in a degraded form.’ (Bernal, 1951; Wigner, 1961)
This may also be expressed in the form of the equation of continuity for entropy (Denbigh, 1951).
δ dS/dt + div S = Θ
Where Θ is the rate of internal creation of entropy, δ the density and S the entropy; div S is the outflow of entropy and δ dS/dt the rate of change of entropy in the enclosed region; Θ must by second law be zero or positive; the possibility that div S can be large and positive makes possible a negative trend for δ dS/dt.
This classification is broad and includes also phenomena such as vortices and flames and many others. Life differs from such primitive processes of the abiological steady state in the singularity, persistence and size of the entropy reduction it sustains. Although limited, this phenomenological description of the class of process, which includes life, is helpful in our search for proof of the existence of Gaia in two ways:
- firstly by serving to define the boundary of the internal region where entropy is reduced, and
- secondly by suggesting that the recognition of a living entity can be based upon the extent of its physical and chemical disequilibrium from the background environment.
On the matter of boundaries, it is obvious that a man, as an example of a living entity, takes in free energy in the form of the chemical potential difference between food and oxygen and susstains a low internal entropy through excretion of waste chemicals and heat. To a man, the environment to which entropy is discarded includes the atmosphere and his boundary is therefore his skin. It might seem pointless therefore on Earth to seek the existence of a general living system, Gaia, in terms of entropy reductions within the atmosphere which clearly for some species is a sink for degraded products and energy but this neglects the fact that photosynthetic life uses premium radiation direct from the sun to sustain a high chemical potential gradient within the atmosphere on a planetary scale. For a tree, the boundary within which entropy is reduced is not its surface in contact with the atmosphere but rather the interface between the sun with the atmosphere as an extension of the tree. The tree produces not only food for consumers but also the equally important gas, oxygen which does not accumulate within the tree waiting to be eaten.
When the whole assembly of life is so seen it is clear that the true boundary is space. The outgoing entropy flux from the Earth indeed from Gaia ‘if she exists’, is long wavelength infra red radiation to space. This then, is the physical justification for delineating the boundary of life as the outer reaches of the atmosphere. There is also to a lesser extent an inner boundary represented by the interface with those inner parts of the Earth as yet unaffected by surface processes. We may now consider all that is encompassed by the bounds as putative life. Whether or not Gaia is real will depend upon the extent to which the entropy reduction within a compartment such as the atmosphere is recognisably different from the abiological steady state background.
On the matter of recognition a debt is owed to the fertile concept of information theory, Shannon & Weaver (1963). It has been demonstrated, for example by Evans (1969) that the classical properties, entropy and free energy, have exact information theoretic equivalents. Thus the information (I) of a system can be defined as
I=S0 – S
where S0 is the entropy of the components of the system at thermodynamic equilibrium and S the entropy of the system assembled. This relationship can be transferred directly from information theoretic to classical thermodynamic terms as follows:
I = (E +PV – TS – ΣN, Ni)/T
where the right hand side of the equation expresses information in terms of temperature (T) pressure (P) internal energy (E), volume (V), entropy (S), and the chemical potential (N) of the molecules present; it follows that information is a measure of disequilibrium in the classic sense and recognisability in the information theoretic sense.
Table 1. The partial pressures in millibars of CO2, N2 and O2 on Mars, Earth and Venus and on two model abiological Earths.Model (D) after life had been deleted and Model (I) a geometric mean interpolation between Mars and Venus
Abiological earth models Gas Venus Earth Mars D I CO2 90,000 0.3 5 0.3-1,000 300 N2 1,000 780 0.05 1 30 O2 0 210 0.1 1 0.3
By examining the extent to which the atmosphere is in chemical and physical disequilibrium both within itself and with the surface of the Earth we have a measure of the extent to which it is recognisable as a separate identity against a neutral background equilibrium state. Whether or not it is seen to be a component part of Gaia will depend upon the size of the disequilibrium revealed.
Thermodynamic evidence
We can determine the degree of departure from a conceivable abiological steady state atmosphere of the Earth by two means; firstly, by comparing the Earth’s atmosphere with Mars and Venus which may be taken as representative of lifeless planets and secondly by comparing the present atmosphere with the atmosphere to be expected if life were deleted from Earth.
Table 1 shows the atmospheric composition of Mars and Venus compared with that of a hypothetical abiological Earth interpolated between them. Also shown is the atmospheric composition to be expected of the Earth if life were deleted. This was derived as follows: nitrogen reacts under the influence of solar UV, electrical discharges, ionisation radiation and combustion with both O2 and CO2 to give oxides of nitrogen. By such processes in time all nitrogen would be converted to the stable NO3– ion dissolved in the oceans. At the pE even of Mars the reverse reactions converting NO3– to N2 do not proceed. In the course of the removal of N2 most of the O2 also would go from the atmosphere. The continuation of water and CO2 photolysis would ensure the production of some oxygen, so that nitrogen removal could go to completion. In the end the atmosphere would apart from argon be dominated by CO2 like those of Mars and Venus.
The model of a lifeless Earth coincides well with that of a planet interpolated between Mars and Venus, the slight chemical disequilibrium is due to dissociative effect of sunlight and the escape of hydrogen. The present Earth is clearly recognisable against the background of the lifeless model.
The terrestrial planets orbit in the outer reaches of the sun’s hydrogen atmosphere and their interiors are reducing hence any concentration of oxygen in a planetary atmosphere is indicative of disequilibrium. Mars has 0.01% of oxygen in its atmosphere and Venus less than this. Such small oxygen concentrations are quite consistent with the abiological steady state consequent upon the upper atmosphere photolysis of water vapour and CO2 on Mars and perhaps to a lesser extent on Venus. It is well to consider that Venus has probably lost oceans of water by photolysis and other reactions, yet still has no O2. The presence of nitrogen on Venus does not indicate disequilibrium since Venus is both hot and acid and bereft of water, these are all conditions favouring nitrogen rather than NO3– as the stable compound. That nitrogen has not yet been detected on Mars is in agreement with the expectation of equilibrium chemistry. A lifeless Earth would similarly be without atmospheric nitrogen. These arguments were used successfully to predict that Mars would lack N2 before this fact was discovered (Lovelock & Giffen, 1969).
The anomalies of abundance are more marked with the lesser gases as is shown in Table 2 which lists their concentrations, residence times, sources and expected concentrations, were chemical equilibrium determining.
Table 2. Gases in the Earth’s atmosphere with origins in the BiosphereThe source strengths, residence times, concentrations, and concentrations expected if the atmosphere was a thermodynamic equilibrium
Gas Atmospheric abundance Anticipated equilibrium concentration Departure from equilibrium Residence time years Output megatons Yr-1 Source N2 0.8 E-10 E10 3E6 E3 Denitrifying bacteria CH4 1.5E-6 E-35 E29 7 2E3 Anaeobic fermenting bacteria N2 3E-7 E-20 E13 10 6E2 Denitrifying bacteria NH3 1E-8 E-35 E27 E-2 1.5E3 Nearly all organisms CH3I 1E-12 E-35 E23 E-3 30 Marine algae
The simultaneous and large fluxes of N2 CH4, NH3, N2O and O2 are all inconsistent with an abiological model. The degree of departure from equilibrium expectations is to be measured in 10’s of orders of magnitude. In these circumstances there is almost a certainty that the atmosphere is a part of the biosphere, for nowhere, but in living systems is so intensive and constant a disequilibrium revealed. Conversely there is a neglible possibility that the atmosphere is a neutral background source of materials which life merely recycles.
The atmosphere as a contrivance
If we assume the Gaia hypothesis, and regard the atmosphere as a contrivance, then it is reasonable to ask what is the function of its various component gases. Outside the Gaia hypothesis such a question would rightly be condemned as circular and illogical but in its context such questions are no more unreasonable than asking, for example, what is the function of fibrinogen in blood. The function of oxygen and nitrogen in sustaining the cycle of raw material and energy is evident. Less obvious is the function for example of CH4. This gas is made on a scale requiring the shunting of as much as 5% of the photosynthetic energy of the entire biosphere. In the Gaia context it must surely have an important purpose of biology is normally efficient and parsimonious over waste. Such a possible important function for methane has been proposed (Lovelock & Lodge, 1972). It is seen as a kind of molecular hydrogen balloon whose purpose is to carry excess hydrogen to the upper atmosphere where it can escape and thus sustain the present oxygen tension of the Earth. The details of this notion are given in the reference quoted. Wofsy et al., (1972) suggest that such is the magnitude of the upward transfer of methane to the stratosphere where it is oxidised to water vapour that the stratosphere is a source of water vapour to the troposphere rather than a sink. It follows that much of the water vapour in the upper atmosphere which is photolysed to produce oxygen is a biological product resulting from the oxidation of biological methane.
As with CH4 the biosphere uses a great amount of energy for NH3 production. When the total production of acids by the oxidation of nitrogen and sulphur is taken into account the ammonia production by the biosphere is found to be just sufficient to sustain a rainfall pH near 8, the optimum for life. One penalty of oxidising environments is the tendency to low pH as the elements such as carbon, nitrogen and sulphur oxidise. The huge biological ammonia production conveniently answers this need. Can it be accidental and have always been so? Other atmospheric gases and vapours for example dimethyl sulphide, dimethyl selenide and methyl iodide may serve in the mass transfer of essential elements between the land and the sea.
A gas wholly in disequilibrium, a biological product on a grand scale, is nitrous oxide. It is made by soil microorganisms and some penetrates to the stratosphere where it reacts to give NO2. By the Gaia hypothesis it must have an important atmospheric purpose, could this be concerned with the regulation of the position or density of the ozone layer? In the summary there is overwhelming evidence that the atmosphere apart from its content of noble gases is a biological product. It may also be a biological contrivance; not living but as essential a part of the biosphere as is the shell to a snail or the fur to a mink.
Systems analysis
One set of tests which can be applied to prove the existence of a control system are those which make changes in the variables thought to be controlled. It is possible to show for example that the core temperature of a man remains constant when the environmental temperature ranges between 0 and 45°C. With the Earth it is reasonable to suspect that at least the following variables may be controlled: (1) Atmospheric, oceanic and soil chemical composition. (2) Surface temperatures in the ‘core’ region i.e. between 45° N and 45° S latitudes. (3) Surface and ocean pH.
It is not usually possible to vary the global environment as a test but perturbations affecting these variables have accompanied the evolution of the solar system. Since life began, 3.5 x 109 years ago, the solar output has probably increased between 30% and 100% (Sagan & Mullen, 1972). The most recent estimates in which the current low rate of neutrino emission from the sun is taken into account is 70% (Dilke & Gough, 1972). It is even suggested that in addition to the slow exponential increase in solar output, fluctuations of 10% may occur over short periods i.e. 10 M years.
The other major environmental change is that of redox potential. At its formation the Earth was highly reducing with hydrogen as a predominant atmospheric gas. It is suggested that 3.5 x 109 years ago the Earth’s pE was -5 at which gases such as CH4, NH3, H2S etc. would be stable in their own chemical right. Hydrogen would be escaping to space but for some time there would be sufficient to regenerate the hydrides dissociated by solar UV. Such an atmosphere could according to Sagan & Mullen (1972) effectively retain heat. It would permit surface temperatures similar to those of present in spite of a much reduced solar output. This is due principally to the radiative properties of gases such as ammonia and the others mentioned above. These were probably the conditions under which life evolved.
Soon after the evolution of life and possibly because of it, Yeas (1972) the pace of hydrogen loss accelerated. By 3.2 x 109 years ago the pE had risen above -4 at which level NH3 is no longer stable in its own right (Sillén, 1966). Had the laws of chemistry and physics operated soon after this time NH3 and other polyatomic reducing gases would have vanished leaving an atmosphere of nitrogen and CO2 at a concentration not substantially different from the present. If we assume that the solar output was in fact lower at this time such an atmosphere could not possibly have sustained present day temperatures the short term equilibrium temperature would have been -5 and – 6°C. If the large albedo of ice and snow cover is additionally taken into account the equilibrium temperature could even have been lower.
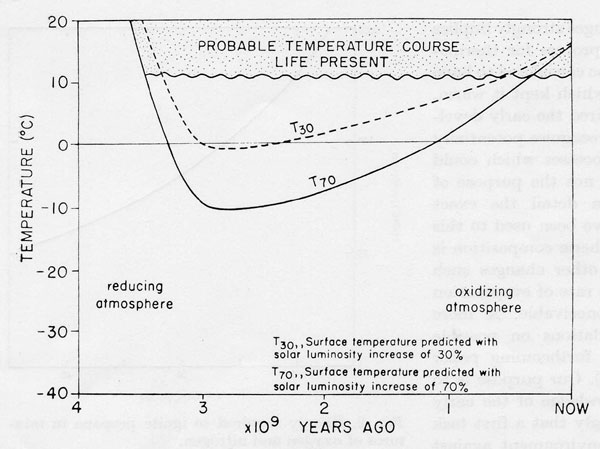
Figure 1. The mean surface temperature of the Earth to 3.5 x 109 years B.P. Shaded area, temperature range deduced from the geological record and the persistence of life. Solid and dashed lines, the temperatures expected for a lifeless Earth assuming a 70% (solid line) or a 30% (dashed line) increase in solar output.
Chemical changes of the evolving earth and the physical changes of its radiation environment are shown in Fig. 1. The geological record and the fact of the persistence of life provide strong evidence that at no time in the past 3.5 x 109 years did the oceans freeze or the mean temperature rise above 50°. Indeed the evidence suggests that apart from glacial episodes, the temperature has always been constant. Glaciations do not usually affect regions between latitudes 45° N and 45° S where 70% of the surface resides.
Sagan & Mullen (1972) propose that the continuous biological synthesis of ammonia before oxygen appeared gave an atmospheric concentration of ammonia sufficient to sustain by its greenhouse effect equable and tolerable temperatures. This is supported by the fact that the current biological ammonia production, which is 2 x 109 tons per year, might in a neutral or reducing atmosphere have sustained the concentration of ammonia said to be needed to provide the desired radiative properties namely 10-5 by volume. However, if we assume that the solar output was indeed lower in remote times, the mere blind production of a gas such as NH3 by the biosphere would not have been likely to suffice in the control of surface temperatures by its radiative properties. With the reduced solar output and the possibility of positive feedback on cooling, any failure of the ammonia harvest or the evolution of a vigorous NH3 consumer, of which there are many today, would have set in train a course for catastrophic irreversible cooling. A mechanism which plausibly can oppose an unfavourable trend is not enough, there must also be a system able to sense the trend and actively control the means for its opposition.
It must be observed that there are other ‘greenhouse’ gases than NH3. CO2 a at high concentrations could have performed a similar function as could other polyatomic gases. To sustain a constant temperature for 3 x 109 years in the face of chemical and physical environmental changes strongly implies the presence of an active process for thermostasis. Life at its origin can be considered to have fed on the blanket of gas which kept it warm. Its continued survival required the early development of the capacity to recognise potentially adverse changes and of processes which could oppose such changes.
It is not the purpose of this article to consider in detail the exact mechanism which could have been used to this end. The change of atmospheric composition is a powerful technique but other changes such as in the surface albedo the rate of evaporation of water, are at least conceivable. A more detailed account of speculations on possible mechanisms is given in a forthcoming paper (Margulis & Lovelock, 1973). Our purpose is to stress that the present knowledge of the early environment suggests strongly that a first task of life was to secure the environment against adverse physical and chemical change. Such security could only come from the active process of homeostasis in which unfavourable tendencies could be sensed and counter measures operated before irreversible damage had been done.
In addition to the possibility of remorseless environmental change set by the evolution of the sun, there were also major changes in the chemical environment. These were caused in part by the evolution of life itself but they also reflected upon the physical environment through the change in atmosphere composition. Prominent amongst these changes must have been the first appearance of free oxygen in the atmosphere which is thought to have taken place between 1 and 2 x 109 years ago.
It is worth digressing a moment to consider what a catastrophic air pollutant oxygen must have been. Its presence in the air must have destroyed a vast range of species and driven underground into anaerobic muds others from which prison they have never returned. Not only was the appearance of oxygen itself a biological crisis but also the rapid reduction of pH which inevitably would accompany oxidation of nitrogen and sulphur compounds and the increased rate of removal of ammonia must have posed other serious problems. Firstly in the maintenance of pH itself and secondly in sustaining a sufficient concentration of ammonia as this gas was depleted by its new role of neutralising acidity.
Again the details of the atmospheric changes of this time are still not known although it is conceivable that the CO2 concentration rose as the pH fell and to some extent offset the effects of the loss of NH3. It is unlikely however that this alone would have been sufficient to sustain tolerable temperatures at this period when the solar output may have been at least 10% and possibly 40% less than now. Nevertheless sufficiently constant temperatures were sustained and life persisted. Even today the regulation of oxygen concentration may represent an important task. Fig. 2 shows how drastically the probability of the ignition of inflammable material rises with increasing oxygen concentration. Above 25% even trees in rain forests might be in hazard from fire. With these crises as with those earlier the blind adaptation to change seems unlikely to have been enough.
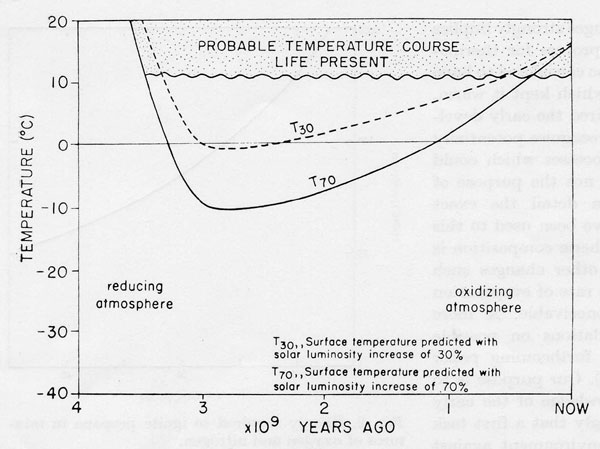
Figure 2. Energy required to ignite propane in mixtures of oxygen and nitrogen.
Conclusions
We have presented some of the evidence concerning the Gaia hypothesis. The hard core geochemists will still no doubt argue that the cycling of gases through the biosphere is a passive process and does not determine atmospheric composition. He may compare it with the cycling of water from the oceans to the land. The delivery of rain to the land surfaces involves a disequilibrium of gravitational and osmotic potential and is driven by solar energy. The vegetation merely borrows the rain and repays at the rate of borrowing. We think that it is all a matter of degree. Sunlight may distil water from the sea later to fall as rain on the land but sunlight does not spontaneously at the Earth’s surface split oxygen from water and drive reactions leading to the synthesis of intricate compounds and structures. The disequilibrium characteristic of water cycling is minor when compared with that of cycling of the atmospheric gases.
To those who are convinced that the atmospheric gases are biological products but are reluctant to accept the notion of homeostasis we say: if life has merely a passive role in cycling the gases of the air then the concentrations will be set by equilibrium chemistry; in fact they most certainly are not. If life actively cycles the gases then we ask how could such a system be stable in the long run without homeostasis? The purpose of this paper is to introduce the Gaia hypothesis at least for entertainment and for the induction of new questions about the Earth. Proof of Gaia’s existence may never approach certainty but further evidence is more likely to come from the study of the contemporary Earth. Astronomical evidence is notoriously fickle and although geological evidence is rather more certain one learns less about a person from the study of his grandfather’s bones than from talking to him face to face.
Acknowledgement
We acknowledge the unstinted help and advice of our colleagues S. R. Epton, P. B. Fellgett, J. P. Lodge and P. G. Simmonds. We thank Shell Research Ltd., for financial support to one of us (J. E. L.)
References
Bernal, J. D. 1951. The physical basis of life. Routledge and Kegan Paul, London.
Denbigh, K. G. 1951. The thermodynamics of the steady state. Methuen and Co., London.
Evans, R. B. 1969. A proof that essergy is the only consistent measure of potential work. Ph.D. Thesis, Dartmouth College, University Microfilms, Ann Arbor, Michigan.
Hitchcock, D. R. & Lovelock, J. E. 1967. “Life detection by atmospheric analysis”. Icarus 7, 149- 159.
Hutchinson, G. E. 1954. “Biochemistry of the terrestrial atmosphere”. In Chapter 8 of The Solar system (ed. Kuiper). University of Chicago Press, Chicago.
Junge, C. 1972. “The cycle of atmospheric gases – natural and man made”. Quart. J. E. Met. Soc. 98, 711-729.
Lewis, G. N. & Randall, M. 1923. Thermodynamics and the free energy of chemical substrates. Chapter 39. McGraw Hill, New York.
Lovelock, J. E. 1972. “Gaia as seen through the atmosphere”. Atmospheric Environment 6, 579- 580.
Lovelock, J. E. & Giffen, C. E. 1969. Planetary atmospheres: compositional and other changes associated with the presence of life, advanced space experiments (Ed. O. L. Tiffany and E. Zaiteff) pp. 179-193. American Astronautical Soc., Washington, D.C.
Lovelock, J. E. & Lodge, J. P. 1972. “Oxygen in the contemporary atmosphere”. Atmospheric Environment, 6, 575-578.
Margulis, L. & Lovelock, J. E. 1973. “Biological modulation of the Earth’s atmosphere”. Icarus, in press.
Sagan, C. & Mullen, G. 1972. “Earth and Mars: evolution of atmospheres and surface temperatures”. Science 177, 52-56.
Schrodinger, E. 1944. What is Life? Cambridge University Press.
Shannon, C. E. & Weaver, W. 1963. The mathematical theory of communication. University of Illinois Press.
Sillén, L. G. 1966. “Regulation of O2, N2, and CO2 in the atmosphere: thoughts of a laboratory chemist”. Tellus 18, 198-206.
Tribus, M. & Mclrvine, E. C. 1971. “Energy and information”. Scientific American 224, No. 3, 179- 188.
Wigner, E. P. 1961. The logic of personal knowledge. Routledge and Kegan Paul, London.
Wofsy, S. C., McConnell, J. C. & McElroy, M. B. 1972. “Atmospheric CH4, CO and CO2“. J. Geophys. Res. 77, 4477-4493.
Yeas, M. 1972. “Biological effects in the early atmosphere”. Nature 238, 163-164.
