This paper by James E. Lovelock and C. E. Giffin was submitted September 1968 to the American Astronautical Society and published in Advances in the Astronautical Sciences, 25, pp.179-193, 1969. It is the first scientific paper to discuss Earth System Science as it is currently understood.
This paper presents the results of one phase of research carried out at the Jet Propulsion Laboratory, California Institute of Technology, under Contract No. NAS 7-100, sponsored by the National Aeronautics and Space Administration.
Author’s note: One achievement of the space programme was that it finally succeeded in convincing geo-planarians that the Earth was not flat; such an enlargement of our view is not restricted to popular mythology; a new view of the Earth from space is an inevitable by-product of all explorations of the planets, whether real or in the imagination. A particular example, which is the subject of this paper, is a clearer view of the Earth’s ecosystem which comes from a consideration of the chemical composition of the atmospheres of Mars, Earth, and Venus.
In 1965, we were interested to know the extent to which information on the chemical composition of planetary atmospheres could constitute direct and primary evidence of life [1]. In other words, could the chemical analysis of a planetary atmosphere constitute a life detection experiment? We convinced ourselves and a few others that such an experiment could indeed detect the presence of life on a planet with a signal to noise ratio at least as good as that of other life detection experiments. At that time, the detection of life on Mars seemed ample justification for this exobiological exercise; as time passed, however, evidence accumulated from the Mariner missions [2] and from the Infrared astronomy of the Connes and Kaplan [3] to suggest that Mars may not be far from an abiological steady-state and therefore unlikely to be a base for life. At this time, therefore, especially in view of the restricted funds available for planetary exploration, it seems relevant to ask: is there sufficient justification for a biologically oriented atmospheric analysis experiment?
The principal purpose of this paper is to provide new arguments and evidence in support of such an experiment and to show that it can be a source of information and ideas towards the comparatively new subject, planetary ecology, with particular reference to the planetary ecosystem which includes all of the life on Earth.
The theoretical basis and the evidence in support of an atmospheric compositional analysis as a life detection experiment has already been given in detail [1] [4]. There follows a brief restatement, modified to include new evidence and oriented towards the planetary ecological viewpoint.
It is a property of an ecosystem to develop to a steady-state at which the supplies of energy and raw materials set a limit. If the living components of the ecosystem are chemical in form then the fluid media, including the planetary atmosphere, may function as a transfer system for the products of the life cycle. This function will modify the composition of the transfer medium so that it is recognizably different from that of an abiological steady-state distribution of chemical compounds.
Confusion often attends attempts to apply the thermodynamic concepts of entropy and equilibrium to living systems and this present topic is no exception. It is generally agreed that it is a property of life to reduce its internal entropy through the assimilation of free energy and the excretion of degraded energy to the environment. Controversy can arise, however, over the size of the maximum unit of life. There is, for example, no doubt that an animal has a highly ordered chemical composition, but its environment to which disorder is rejected includes the atmosphere; it might seem pointless therefore to seek evidence of life by looking for order in the chemical composition of the atmosphere. If instead of individual living organisms, however, the planetary ecosystem itself is regarded as the maximum unit of life, the problem resolves. In an ecosystem, the atmosphere can have an ordered role as the conveyor belt for products between, for example, the plant and animal kingdoms of the Earth, or their analogues elsewhere. With this large unit, the atmosphere is an internal component of the living system, and the environment is now space, to which disorder is rejected in the form of degraded solar energy.
When these arguments are used as the basis of a life detection experiment, it is necessary to demonstrate that no abiological steady-state processes could account for the observed ordered distribution of atmospheric components. In this context, a frequent further source of confusion comes from the use of the term “disequilibrium”. No planetary atmosphere, so long as the sun shines, can possibly be at a state of thermodynamic equilibrium. The comparison of the atmosphere of living and of lifeless planets is a comparison of degrees of disequilibrium; when it is used to detect the presence of life, the certainty of detection, as in other measurements and in other life detection experiments, is a statistical measure and can only approach but not reach unity.
At the time work on this topic was commenced in 1965, it was possible to offer an experimental proof by using the chemical composition of the Earth’s atmosphere as evidence for the presence of life. Thus, the simultaneous presence of the two gaseous components, oxygen and methane at 21 per cent and 1.5 ppm respectively, was sufficient evidence. In the presence of this high concentration of oxygen, there is no possible inorganic process for the production of methane, either in the atmosphere or at the surface. The rate of removal of oxygen by methane oxidation at the observed methane concentration is far greater than oxygen could be produced by photolysis in the upper atmosphere; which is the only possible abiological reaction for the production of oxygen. Even in the highly unlikely event of the out gassing of methane at a rate sufficient to maintain its steady-state concentration against oxidation, it would still be necessary to explain the process for oxygen production. By far, the most probable explanation, therefore, is that both oxygen and it methane are almost entirely maintained at a steady-state concentration by the Earth’s ecosystem.
Since 1965, aeronomic evidence has accumulated to suggest other major and trace components of the atmosphere are also biologically maintained. Nitrogen and nitrous oxide, for example, could not be present at their observed levels solely from inorganic sources. In spite of the recurrent comment [5] [6] [7] that the stable form of the element nitrogen at the pH, pO2, and temperature of the Earth is the nitrate ion, gaseous nitrogen is still often regarded as if it were a stable permanent product of primitive out gassing as inert as the rare gases.
In fact, the residence time of atmospheric nitrogen is less than 108 years. Nitrous oxide is rapidly decomposed by ultraviolet radiation in the stratosphere and there is no abiological source in the atmosphere capable of maintaining its observed concentration of 0.5 ppm [8]. There is, however, ample evidence of its production in sufficient quantities by soil micro-organisms; indeed, it is possible that nitrous oxide may be a principal precursor of gaseous nitrogen in the nitrogen cycle. The abundance of the Earth’s atmospheric components and a comparison of their biological and abiological sources and sinks are listed in Table 1.
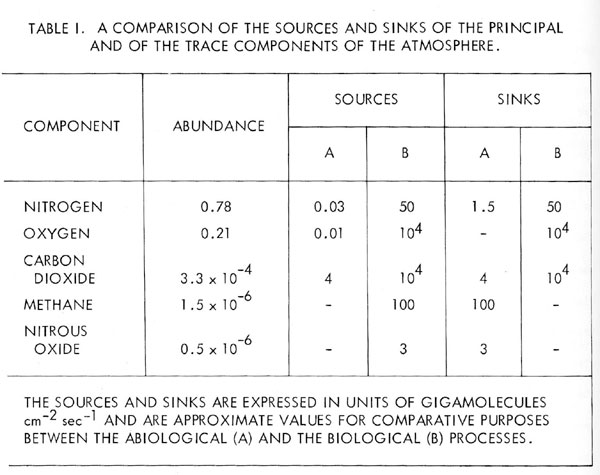
This work was commenced with the goal of a life detection experiment for Mars; in terms of flight hardware, this goal has not been reached. Nevertheless, the search for life by this means has in true serendipity rediscovered an ancient life form; the Earth’s ecosystem or as it used to be known, “Nature”. The change of viewpoint which made this possible is that an atmospheric life detection experiment forces a view of the atmosphere, which is contemporary. For the purpose of such an experiment, the historical origins of the atmospheric gases are not important; what is important are large scale processes which maintain the current steady-state composition. Recondite questions such as: did the biology produce the oxygen or does it merely process the gas in the course of its metabolism? Need no longer vex us. The contemporary view of the atmosphere penetrates the endless controversy surrounding its historical origins. Whatever its past may have been, the air is now revealed as a complex biological contrivance.
The model of the Earth’s atmosphere as a part of a biological controlled system makes an interesting comparison with the atmospheres of Mars and Venus. If these planets are for this comparison taken to be lifeless, then their compositions represent models of the abiological steady-state. Table 2 lists the current views of the composition of the Mars and Venus atmospheres.
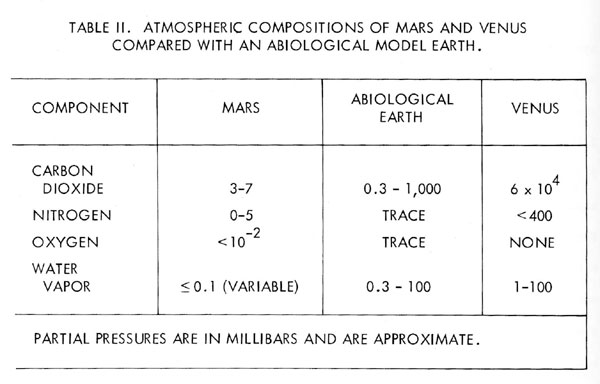
Perhaps the best approach to this comparison is to consider the changes which might occur in the composition of the Earth’s atmosphere were life to cease. The nitrate ion is a stable compound of this element and there are abiological processes for the oxidation of nitrogen in the atmosphere, nitrogen would therefore be expected gradually to leave the atmosphere. Oxygen would also fall in concentration, partly through the oxidation of reducing material continually exposed at the surface and partly through the oxidation of nitrogen and methane. The oxygen concentration would eventually stabilise at some low-level due to the equilibrium between its production by water photolysis and its removal by the processes just listed. The oxygen equilibrium would ensure the continued removal of nitrogen until a new low-level equilibrium of this gas also was established, where the removal by oxidation and the production by out gassing were in balance.
Although the exact course and pathways for the removal of oxygen and nitrogen from the atmosphere are uncertain, the ultimate fate of these gases would be to form stable anions probably mostly in the sea. The remaining atmosphere would be one of argon, carbon dioxide and water vapour with traces only of oxygen and nitrogen. The final abiological steady-state atmosphere of the Earth would depend strongly on factors governing the carbon dioxide concentration. If the carbon dioxide pressure were high, then the temperature and composition would tend towards that of Venus. If the carbon dioxide partial pressure remained as it is now, then that the conditions would be closer to those of the Mars.
These arguments raise the possibility that there is little or no nitrogen in the Mars atmosphere. The thin atmosphere of this planet might in part, at least, be as much a consequence of the inorganic chemistry of oxygen and nitrogen as of a failure of the planet to outgas. If the nitrogen concentration on Venus is confirmed, it could be that Venus is too hot and too acid for the stability of the nitrate ion.
If the atmosphere of the Earth is a biological contrivance, then it is reasonable to consider that the components are maintained at an optimum or near optimum composition for the ecosystem. For example, the Earth’s climate is strongly dependent upon the atmospheric pressure, that is, the total amount of oxygen and nitrogen, and on the concentration of infrared absorbency gases such as carbon dioxide and water vapour. The concentration of these components are directly or indirectly under biological control. It may not therefore be an unreasonable speculation to consider the possibility that the Earth’s climate is also maintained at or near an optimum for the ecosystem.
It is interesting to ask, why is the oxygen concentration maintained at 21%. It is a fact that the energy required for the ignition of organic compounds changes by about 70% for each 1% change in oxygen concentration at the atmospheric level. Life at even 25% oxygen might be very uncomfortable, especially for trees. The removal of oxygen by grass and forest fires may set the upper limit of 21% but it seems more likely that oxygen is actively controlled at a safe maximum. Figure 1 shows the relationship between oxygen concentration and the energy required to ignite a typical organic substance, propane.
Let us now consider the strategy and the hardware of an atmospheric analysis experiment. For a biologically oriented atmospheric analysis experiment, we need to know, with only moderate accuracy, the concentrations of all chemically active gases down to parts per 107 by volume. It would also be helpful to have information on the vertical profiles of the distribution of the gases, and perhaps also information on diurnal variations of some gases at the surface.
No single instrument can provide all the information we need; however, a combination of instruments chosen to be complementary can get close to fulfilling the needs.
Such a complimentary configuration of instruments would comprise an entry mass spectrometer for the analysis of the atmosphere prior to impact followed by a simple gas chromatograph capable of surviving the hard landing, and whose function will be to fill in the blind spots in the entry mass spectra.
Considerable effort has been directed toward the implementation of instrumentation for in situ measurements of the composition of the Mars and Venus atmospheres. It is generally accepted that a mass spectrometer experiment is the best approach for measuring species concentrations in the range of 10-20 ppm to 100%, where a rapid response is mandatory to the accomplishment of the analysis (in the case of Mars, only a few tens of seconds at best are available for an atmospheric compositional analysis experiment carried upon the presently conceived non-survivable entry capsules).
Described below is a mass spectrometer instrument designed by the Jet Propulsion Laboratory for the analysis of the Mars atmosphere from a high velocity non-survivable entry capsule. The instrument is a double focusing, magnetic sector mass spectrometer with a mass range of 10 amu to 90 amu with unit resolving power being achieved at 90 amu. The detectability of the mass spectrometer is 20 ppm and a mass scan over the 10 to 90 amu mass range is accomplished every three seconds.
Figure 2 is a block diagram of the mass spectrometer and associated electronics. It should be noted that the vacuum requirements of the instrument are maintained simply by extending the pole pieces of the mass analyser magnet and incorporating a small ion pump between them. Atmospheric gas introduction is accomplished through a molecular leak to ensure accurate analysis of the atmospheric species.
Figure 3 is a photograph of the packaged version of the mass spectrometer. The black objects are the potted electronics which have been constructed using a three dimensional, welded wire cordwood module techniques. These potted elements serve additionally as structural members for environmental stability. Surrounding the electronics modules is the mass spectrometer itself. The ion source housing can be seen on the right, followed by the 90° electrostatic sector housing, the 60° sector magnet, and finally the electron multiplier detector housing.
The entire instrument when completed for flight will weigh 10 pounds and consume 8 Watts of power including a data handling system. Less than 800 binary bits of information are required to telemeter a mass spectra such as that shown in Figure 4, which comprises a synthetic mixture of krypton, carbon dioxide, argon, oxygen, nitrogen and methane.
The capability to land and survive on the planet’s surface greatly enhances the retrieval of significant scientific information from an atmospheric compositional analysis experiment. In this situation, the constraint of rapid analysis is removed and the usage of gas chromatography becomes practical. As noted above, certain molecular species of prime interest are not uniquely identifiable by their mass number (i.e. molecular weight), and result in blind spots in the entry mass spectra. For example, molecular nitrogen and carbon monoxide both have a molecular weight of 28 amu, carbon dioxide, and nitrous oxide both have a molecular weight of 44 amu, etc.
In addition, disassociation of molecular species in the ion source of the mass spectrometer can contribute fragment ions of gases, which may be desirable to detect in the planetary atmosphere at low concentrations. Consider the case of carbon dioxide, which disassociates in the ion source to produce a carbon monoxide fragment ion. This fragment ion contributes a background at mass 28, which must be subtracted out in proportion to the amount of carbon dioxide present in order to accurately ascertain the true quantity of CO and N2 in the planetary atmosphere.
Likewise, the true CO abundance can only be determined after correction of the 28 mass peak for molecular nitrogen utilising the atomic nitrogen fragment ion at mass 14 as an indicator. In an ideal situation, where there are no errors associated with ion current measurements, virtually any mixture can be analysed through the use of known fragmentation patterns in a set of simultaneous equations. However, in the real case where errors are associated with the ion current measurements at each maths, the overlapping of spectra creates a lower limit of detection on certain species, which is dependent on the atmospheric composition itself.
The seriousness of the problems cited above increases as one attempts to analyse smaller and smaller constituent concentrations. The obvious solution to the problem of trace constituent analysis is the use of a gas chromatograph where gaseous separation is affected not by mass but rather by the reversible absorption of the sample species on a particular substrate material. The elution times are characteristic of their molecular properties. The time required for an analysis of the Mars atmosphere, for instance, would be in the order of one minute (which would preclude the use of this instrument in a non survivable, high velocity entry capsule).
An illustration of a gas chromatograph is not included in this paper. This is not because it is merely an idea, but because gas chromatography is an art of such rapid development that it would be quite imprudent to freeze the design until the last possible moment. In fact, the basic hardware of a hard landing gas chromatograph has already been constructed and evaluated at the Jet Propulsion Laboratory. The inclusion of the preferred column and detector components does not involve major changes. The task of the gas chromatograph would be to measure the following gases and their diurnal variations down to parts per 106by volume: hydrogen, helium, oxygen, nitrogen, carbon monoxide, and perhaps nitrous oxide.
In addition to the flight instrumentation, it must not be forgotten that further atmospheric compositional data may become available from Earth based infrared spectroscopy. A double check on the CO content of the Mars atmosphere is in this way available. It is worth noting that with this technique, Lewis Kaplan has revealed the unexpected gases HCl and HF in the Venus atmosphere.
A well designed complementary instrument package of the type described not only provides the information needed to answer the biological questions, but also provides the information needed by the planetologist. It is important to observe that the reverse is not so, and an atmospheric analysis experiment designed for planetological purposes does not usually provide information useful for biological purposes.
The planetary exploration programme has already contributed in many ways to a better understanding of the Earth as a planet. If it can succeed in revealing in full detail the biological cybernetics system of the Earth’s atmosphere, this would seem to be a role nearly as important as the discovery of life elsewhere. Even if the other planets, Mars and Venus, show no evidence of life, their composition and properties still represent important reference points of abiological steady-state equilibria against which the living Earth can be compared.
Such a role may be timely for already on Earth we are within sight of major ecological problems of global dimensions which involves the atmosphere. The present day use of fossil fuel requires 15% of biological production of oxygen; at a growth rate of 5% for industry, in three or four decades fossil fuel burning may be the major sink for oxygen. We cannot be certain that the ecosystem will maintain a status quo favourable for us in the face of so large a perturbation. Oxygen is only one of several atmospheric components similarly affected by industrial activity. Perhaps these problems are technologically soluble. If they are, then a view of the Earth from space will almost certainly help. Therefore, as a foreign visitor to this meeting, I view with dismay the cut back of the modest NASA planetary exploration programme. The potential value of the information and ideas it can provide for these urgent ecological problems ahead make its current cost seem to be quite trivial.
Literature cited
- Hitchcock, D. R., and Lovelock, J. E., Icarus 7, 149, 1967.
- Mariner missions.
- Kaplan, L.D., Science Journal 3, 64, 1967.
- Lovelock, J. E., and Hitchcock, D.R., Science Journal 3, 56, 1967
- Lewis, G. N., and Randall, M., Thermodynamics and the Free Energy of Chemical Substrates, Chapter XXXIX, McGraw Hill, New York, 1923.
- Hutchinson G. E., Biochemistry of the Terrestrial Atmosphere in chapter 8 of the Solar System, Kuiper (ed.) University of Chicago Press, Chicago, 1954.
- Sillen, L.G., Tellus XVIII, 198, 1966.
- Bates, D. R., and Hayes, P. B., Planet Space Science 15, 189, 1967.
